Authors:
Mirna Waked – Saint George Hospital University Medical Center, Beirut, Lebanon
George Khayat – Faculty of Medicine, Hotel-Dieu de France Hospital, Saint-Joseph University, Beirut, Lebanon
Pascale Salameh – Faculties of Pharmacy and Public Health, Lebanese University, Beirut, Lebanon
Background:
Chronic obstructive pulmonary disease (COPD) continues to increase worldwide. The objective of this study was to determine the prevalence of COPD in Lebanese adults. Methods: A cross-sectional study was carried out using a multistage cluster sample from all over Lebanon. Residents aged 40 years and over were enrolled. Subjects underwent baseline spirometry and answered a questionnaire. After an albuterol + ipratropium bromide bronchodilator, a posttest was performed.
Results:
Of 2201 individuals, only 33.3% had never smoked. The prevalence of COPD by the Global Initiative for Chronic Obstructive Lung Disease definition, was 9.7% (95% confidence interval [CI]: 8.5%–10.9%). According to the 5% lower limit of normal definition of COPD, the prevalence was 12.5% (95% CI: 11.2%–13.9%). A total of 20.2% were already diagnosed by a physician. No differences in symptoms across stages of COPD were found, but there was a significant trend for a higher number of visits to the emergency room and to the doctor (P , 0.001), and a higher number of hospitalizations (P , 0.001). Older individuals had an increased risk of COPD (adjusted odds ratio [ORa] = 1.05); so did “ever” cigarette smokers (ORa = 4.88) and water-pipe smokers (ORa = 2.53).
Conclusion:
This is the first epidemiological study in Lebanon that determined COPD prevalence and the link with water-pipe smoking.
Introduction
Chronic obstructive pulmonary disease (COPD) is projected to rank third as a cause of mortality by 2020,1 and its burden is increasing worldwide. Early diagnosis of this disease is a challenge for the coming years.1,2 Risk factors and the clinical context are important for a positive diagnosis, but pulmonary function tests are needed for confirmation.1–3 Defining COPD on the flow volume curve relies on one criterion: the post-bronchodilator forced expiratory volume at 1 second (FEV1)/forced vital capacity (FVC) ratio being less than 70%.1,3,4 New insight has been put on the limitations of such a diagnostic criterion,4–6 and some have discussed the value of the 5% lower limit of normal (LLN) for more accurate COPD diagnosis.4,7 Despite the background outlined above, and the controversy about the changing prevalence of COPD worldwide,8,9 data are still lacking in Lebanon. It is noteworthy that while a smoking ban was finally brought in to public places in Lebanon in August this year, this study would be the first national cross-sectional one with the objective of determining the prevalence of COPD in the Lebanese adult population. It can therefore be a starting point for building health policies for smoking-related diseases in this country.
What was already known on this subject?
No data were previously available about COPD in Lebanon. This is the first national study on adults aged 40 years and over, with no exclusion criteria, revealing a high prevalence of COPD. It highlights also in this smoker population that 80% of the cases are not recognized and not diagnosed as COPD. It reveals a robust link between water-pipe smoking and COPD.
What does this study add?
Raising awareness among health care professionals and the public about COPD is fundamental. This study will encourage early diagnosis of COPD, early treatment, and secondary and primary prevention. This will contribute to the application of the Framework Convention on Tobacco Control about the antismoking policy signed by Lebanon. It will help to demystify the myth about the nonharmful effect of water-pipe smoking.
Methods
Study design and population
A cross-sectional study was carried out between October 2009 and September 2010, using a multistage cluster sample from all over Lebanon. Lebanese residents aged 40 years and over were enrolled in the study, with no exclusion criteria. The reason for choosing 40 years as the lower cutoff age was to reduce the probability of asthma being the prevalent disease in a younger sample. The total number of males in the study population was 614,564, while the total number of females was 653,751.10 The Institutional Review Board of the Lebanese University stated that an approval was not necessary since the study was an observational one and not experimental, clinical, or interventional. No approval number was allocated for this statement.
Procedure
From the list of circumscriptions in Lebanon (villages, towns, and cities),11 100 were randomly selected through a representative of local authorities; randomization was performed using a software program. Individuals aged 40 years and over were then randomly chosen to be interviewed from a provided list of dwelling households. After an oral informed consent, subjects underwent baseline spirometry (Micro Lab; Micro Medical Ltd, Kent, UK) by a trained technician and answered a questionnaire. Since albuterol or ipratropium are recommended bronchodilators to be used for spirometric reversibility testing performed in a laboratory,12 with no clear consensus whether to use both of them simultaneously or consecutively, post-bronchodilator spirometry was performed 30 minutes after the inhalation of two puffs of combined ipratropium bromide (18 µg/actuation) and albuterol sulfate (103 µg/actuation) (Combivent®; Boehringer Ingelheim, Ingelheim, Germany) in a pressurized metered-dose aerosol unit. The best result out of three trials was taken into account. The questionnaire of the American Thoracic Society for evaluation of chronic pulmonary disease13 and the Medical Research Council (MRC) score to evaluate dyspnea 14 were used. Questionnaires were translated into Arabic by an independent translator and checked by investigators; a back translation by another translator was done to ensure lack of discrepancy between English and Arabic versions.
The questionnaire was then pretested in a pilot sample of 20 individuals for finalization of details. Moreover, questions about weight, height, and a diagnosed cardiac problem were also asked. In addition to health questions, data were collected concerning sociodemographic characteristics (eg, age, sex, education, marital status, region of dwelling) and cigarette and water-pipe smoking history (current-, previous-, or never-smokers). Cigarette smoking was defined as smoking more than one pack in a lifetime, while current water-pipe smoking was defined as a positive answer to the question “do you regularly smoke water-pipe?”, and previous water-pipe smoking was defined as a positive answer to the question “were you a regular smoker of water-pipe?”
Sample size calculation
A minimal simple random sample size of n = 1015 was required to measure the prevalence of COPD in Lebanon, in an adult population aged 40 years and over. According to other studies in the world that show a variation of COPD prevalence around 9%–12%,15,16 a worst acceptable result of ±2% difference with the abovementioned prevalence and a 95% confidence interval (CI) was taken into account and added to the multistage sampling design, thus giving a minimal sample size of 2030 individuals.
Statistical analysis
SPSS Statistics (IBM Corporation, Somers, NY) software, version 17.0, was used to enter and analyze data. Weighting was performed according to the numbers published by the Lebanese Ministry of Social Affairs and the Central Administration of Statistics in 2007, taking into account gender, age, and dwelling region.10 Cluster effect was taken into account, according to Rumeau-Rouquette and collaborators.17
COPD was defined and classified according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines,1 and according to the LLN (FEV1/FVC post-bronchodilator below the fifth percentile of the healthy population having the same age and gender of the individual would be considered to have COPD).7 The latter equations were calculated according to the method described by Cole and collaborators, separately for males and females,18 to predict the expected fifth percentile for every individual’s healthy population according to age and gender.
Significant reversibility was defined as an increase in post-bronchodilator FEV1 from baseline by 12% or more and by 200 mL or more.12
The chi-square test was used for cross tabulation of qualitative variables in bivariate analysis, and prevalence ratios (PRs) were calculated. Somers’ d-test was used to evaluate trends between ordinal variables. A backward stepwise likelihood ratio logistic regression was performed for multivariate analysis: COPD was the dependent variable; sociodemographic characteristics (age, sex, marital status, working status, dwelling region, and education) and cigarette and water-pipe smoking were the independent variables. After ensuring model adequacy using the Hosmer–Lemeshow test, adjusted odds ratios (ORa) were calculated. Moreover, a stepwise descendant multiple linear regression analysis was performed to evaluate predictors of obstruction severity (percentage of FEV1 over-predicted) in ever-smokers. Sociodemographic data, cigarette and water-pipe quantitative-related duration, and frequency of exposure were used as independent variables. Linearity of the relationship, normality of residuals, and noncolinearity of retained variables were insured before the model was accepted.
Results
Sample description
Of the 3000 selected individuals aged 40 years and over, 2201 individuals were interviewed and underwent two adequate spirometric measurements (73.4%). After weighting, individuals were grouped into Lebanese regions as follows: 361 in Beirut (16.4%), 882 in Mount Lebanon (40.1%), 407 in North Lebanon (18.5%), 298 in South Lebanon (13.6%), and 252 in the Bekaa Valley (11.5%). There were 48.4% males and 51.6% females. Age groups were as follows: 40–44 years (19.2%), 45–49 years (15.6%), 50–54 years (14.3%), 55–59 years (13.3%), 60–64 years (12.4%), and .65 years (25.1%).
In this sample, 33.3% had never smoked, whereas 60% had ever smoked cigarettes, and 16.3% had ever smoked water-pipe. For current-smokers, 33.2% smoked cigarettes, 6% smoked water-pipe, and 4.2% were current mixed-smokers. For previous-smokers, 20.4% smoked cigarettes, 3.1% smoked water-pipe, and 3.6% were previous mixed-smokers. Of the “ever” cigarette smokers, 15.9% were also “ever” water-pipe smokers; of the “ever” water-pipe smokers, 58.5% had ever smoked cigarettes.
COPD prevalence and staging
Among 2201 individuals, the prevalence of COPD was 9.7% (95% CI: 8.5%–10.9%), according to the GOLD definition. Only 20.2% of these COPD patients had already been diagnosed and treated by a physician. Distribution of COPD stage in COPD patients was as follows: 17.6% mild (stage I), 58.3% moderate (stage II), 20.3% severe (stage III), and 3.8% very severe (stage IV). These figures translate into 1.7%, 5.6%, 2%, and 0.4% of the general population, respectively. Subsequent stage groups included 47.4%, 37.9%, 25%, and 25% of females, respectively; they also included 86.5%, 89.6%, 95.3%, and 100% of ever-smokers, respectively.
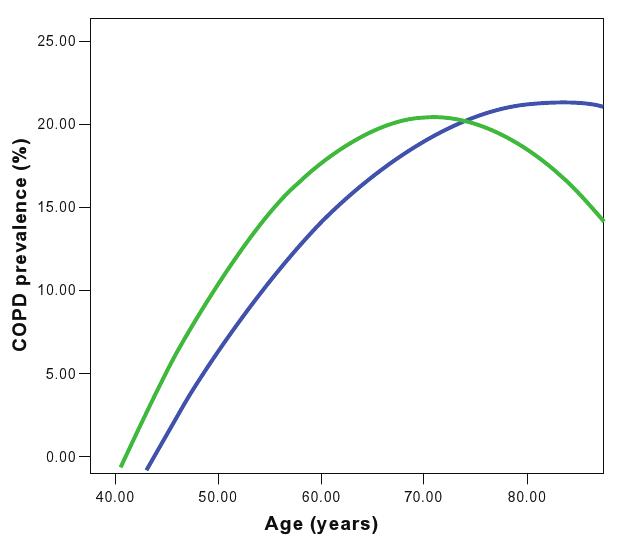
According to the 5% LLN definition of COPD, the prevalence turned out to be 12.5% (95% CI: 11.2%–13.9%); 4.1% of those aged less than 70 years, classified initially as non-COPD, were readjusted as COPD patients according to the LLN 5% definition. The opposite was the case for people aged over 70 years: 11.4% classified as COPD sufferers by the GOLD definition were found to be non-COPD by the LLN 5% definition (Figure 1).
Of the entire sample, 50.2% had no chronic respiratory diseases or symptoms, with normal spirometry; whereas 5.6% had significant post-bronchodilator reversibility on spirometry, with clinical features suggestive of asthma, 12.9% had restrictive pattern, and 22.1% had miscellaneous symptoms and abnormalities on spirometry.
Respiratory symptoms
In this study’s COPD population, the most reported respiratory symptoms were: 52.7% chronic cough (95% CI: 46.8%–58.5%), 59.6% sputum production (95% CI: 53.8%–65.3%), and 60.3% wheezing (95% CI: 54.6%–66.0%). As for dyspnea, the mean MRC score for these individuals was 2.36, the standard deviation was 2.07, and the median was 2.00. The 25th percentile was 0, while the 75th percentile was 5.00.
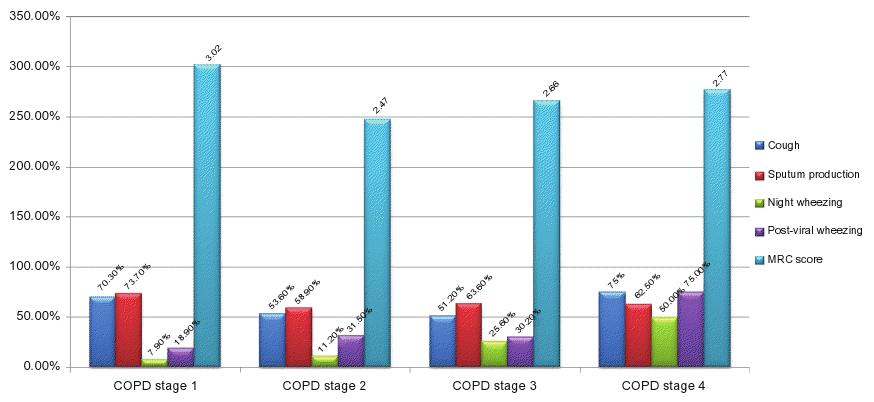
No significant differences in symptoms across stages of COPD were found for chronic cough, sputum production, and dyspnea at rest and on minor, moderate, or major effort. However, a significant trend of ascendance was found for nocturnal wheezing (7.9%, 11.2%, 25.6%, and 50%; P = 0.001) and post-viral wheezing (18.9%, 31.5%, 30.2%,and 75%; P = 0.045), reported in Figure 2A where symptoms are plotted by COPD stage.
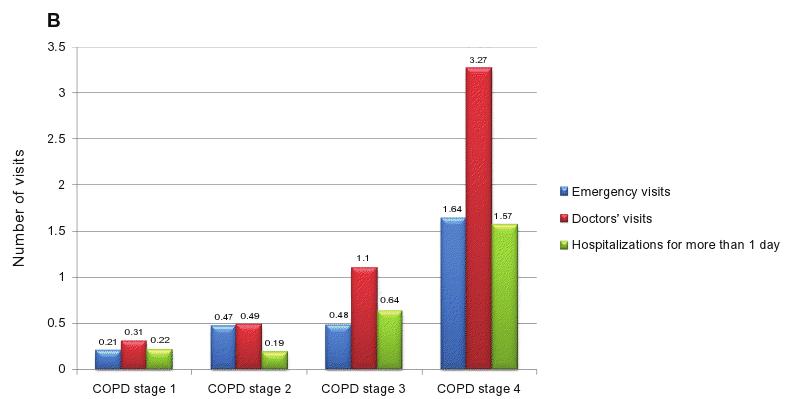
A significant trend was also found across stages of COPD for a higher number of visits to the emergency department (ED) (P , 0.001) and to the doctor (P , 0.001) and a higher number of hospitalizations for more than 1 day due to exacerbations of respiratory problems (P , 0.001), represented in Figure 2B (plotted by COPD severity stage).
COPD bivariate analysis
In Table 1, COPD distribution is reported according to sociodemographic characteristics. There were significant differences between all subgroups: dwellers of the Bekaa Valley and Beirut, males, older age, not currently working, and widows or divorced were subgroups with a higher prevalence of COPD than the other subgroups. They also have a higher risk of cardiac diseases, and they may be more likely to have a low body mass index (BMI).
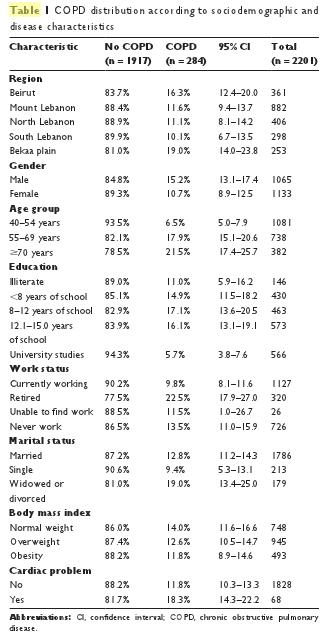
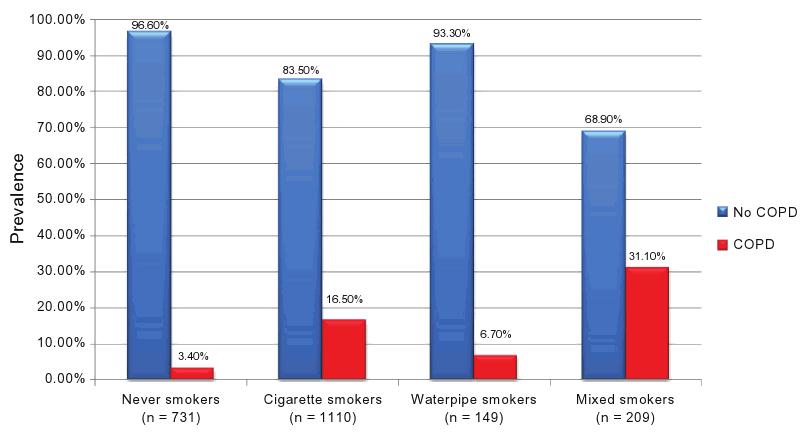
In Figure 3, the COPD prevalence by smoker subgroup is reported. It is noteworthy that the highest prevalence is found in mixed-smokers (31.1%; PR = 9.15), followed by cigarette smokers (16.5%; PR = 4.85), water-pipe smokers (6.7%; PR = 1.97), and finally, never-smokers (3.4%; reference category). Moreover, a significant dose–effect relationship was found for both cigarettes and water-pipes: COPD prevalence increased from 5.2% in non-cigarette smokers to 7.3% in cumulative smokers of less than 15 pack-years, 13.7% if cumulative smoking was between 15 and 45 pack-years, and 34.3% if cumulative smoking was higher than 45 pack-years (P , 0.001 for trend). For cumulative water-pipe smokers, COPD revalence was 11.3% in non-water-pipe smokers, 11.6% in smokers of less than 15 water-pipe-years, 18.2% if cumulative smoking was between 15 and 40 water-pipe-years, and 37.2% if cumulative smoking surpassed 40 water-pipe-years (P , 0.001 for trend).
Multivariate analysis
The multivariate analysis that was carried out for all individuals in this study is reported in Table 2: older individuals have an increased risk of COPD (ORa = 1.05); so do the “ever” cigarette smokers (ORa = 4.88) and the water-pipe smokers (ORa = 2.53). A borderline effect was found for the Bekaa region versus Beirut. All other sociodemographic characteristics were removed from the model.
For nonsmokers, only older age (ORa = 1.23) and having a family history of chronic respiratory diseases (ORa = 2.96) were predictors of COPD. Gender, marital status, education, work status, dwelling district, and cardiac diseases did not significantly predict COPD in this group. Moreover, predictors of obstruction severity in ever-smokers were: a higher age group, a higher cigarette-dependence score, and a longer duration of previous cigarette and water-pipe smoking. Other variables, particularly current smoking-related ones, did not predict obstruction severity (Table 2); whereas, in neversmokers, only older age showed a significant prediction of obstruction severity (beta = −1.77).
Discussion
This is the first national study on COPD prevalence; it reveals a proportion of 9.7% of cases according to the GOLD definition in the adult Lebanese population aged 40 years and over. This prevalence rate is comparable to others reported in the literature and to European countries.15,16,19,20 There is a lack of comparative longitudinal studies, conducted locally, whereas Vasankari et al comparing prevalence of COPD throughout a decade found no increase in the prevalence trend in Finland,21 and Soriano et al found an unexpected decrease in COPD prevalence in Spain.15 The Lebanese data from this present study will be available as a baseline tool for future studies of the impact on smoking-related diseases of the implemented ban on smoking in public places.
It has for a long time been difficult to estimate COPD prevalence1,8 because of low awareness of the disease, under diagnosis, variability within age groups, and discrepancies in the studies’ methodologies.9 Eighty percent of COPD patients in this present study’s population were not diagnosed, and this is comparable to other reported data worldwide.15,19 However, one of the main reasons for under recognition of this disease, for which mortality and morbidity rates are increasing around the world, lies within its very own definition and diagnosis. A post-bronchodilator FEV1/FVC ratio of less than 70% in a spirometry test is still the basis of the diagnosis of COPD.1,12,22 In this present study, the special aspect of the performed spirometry was that both adrenergic and cholinergic pathways of bronchial obstruction23 were targeted by administering combined albuterol and ipratropium bromide inhaler (Combivent). This is not the routine procedure recommended in the guidelines;12 however, no real consensus and standardization exists about the type of bronchodilator, the dose, and the time to wait before the post-bronchodilator test is to be performed.23
The method used in this present study allows hypothetically full room for obstruction improvement during 30 minutes before the post dose test;12,23 relying by that on the different peak effect of the two components (albuterol and ipratropium) of the bronchodilator used. However, this could have lead to an underestimation of COPD prevalence in the study population by a better classification based on the significant reversibility criteria into (1) mixed obstructive disease due to COPD and asthma or (2) pure asthma.
However, the emerging new concept that COPD is an accelerated process of lung aging24 has raised many questions about the limitation of the fixed ratio in diagnosing COPD.3–6 Many have suggested relying on the value of the 5% LLN of FEV1 for the diagnosis of COPD.6,7 It is clear that, by the former method, COPD is over-diagnosed in the higher age groups and under-diagnosed in younger age groups.4,6,7 The results of this present study were consistent with this statement.
Considering the GOLD stages classification, moderate (stage II) was the most prevalent (58.3%) in this present study’s population; this result was comparable to what is found in the literature.4,5,20 It is noteworthy that in this study there was also a consistent number of severe stages (20.3%). The symptoms recorded by the COPD patients in this study were mainly chronic cough, sputum production, and wheezing, whereas dyspnea was the major limiting symptom, especially for those complaining of breathlessness on minor effort (34.9%); these were consistent with other prevalence studies.2,5,15,16,25 The mean MRC score of 2.36 in these individuals points out the significant impact of their disease on their quality of life.14,26,27 No significant differences were found across the stages of COPD regarding sputum production, cough, and dyspnea. These results underline the poor predictive value for severity of the reported symptoms in COPD and is consistent with the literature;2,4,6 whereas the significant trend for more visits to the ED and to the doctor and higher number of hospitalizations for exacerbations across the stages of COPD is consistent with the results found in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) study,5 where seriousness of the disease was unexpectedly reflected by the rate of exacerbations only. This finding may emphasize the need in the future to classify COPD more accurately by phenotypic clusters as suggested by Burgel et al.28
Males and older age, not currently working, and widows or divorced were subgroups with higher prevalence of COPD than the others, and that was comparable to other data in the literature.16,25,28 These subgroups had a higher risk of cardiac disease, which is also a known comorbidity in COPD, the only retained criteria in the bivariate analysis being similar to what is found in the literature.28,29 It is of note that the presence of cardiac disease may by itself overestimate COPD prevalence, since congestive heart disease may show airflow congestion by lung congestion. But in Lebanon, the answer about the presence of any cardiac diseases with the questioned individual would mean in the majority of the cases – if positive – ischemic heart diseases. This issue remains to be discussed in other specifically designed studies.
Living in the Bekaa Valley and the capital Beirut were independent risk factors of COPD; environmental measurements of regional noxious pollutant concentrations are needed to further explain this finding.
On the other hand, smoking has been identified as the main cause of COPD.1 In this present study, there were no other exclusion criteria than being under 40 years old. By looking at the smoking status of the 2201 people included, only 33.3% had never smoked. Consequently, the highest prevalence for COPD was found in mixed-smokers (31.1%), compared with 3.4% of COPD in never-smokers. The multivariate analysis results were concordant with other epidemiological studies,2,3,5,16,25 with one particular finding neither clearly nor frequently reported elsewhere in literature:30 the link between water-pipe smoking and COPD. That finding is consistent with other previously demonstrated associations between water-pipe smoking and chronic respiratory symptoms.31
The 3.4% of patients who were nonsmokers with COPD showed in the multivariate analysis the same risk factors as in other studies such as the aging process,24 previous history of respiratory diseases in the family and acquisition of COPD in early life,19,25,32 and also environmental exposure such as wood smoke and passive smoking.16,32 Unlike other reports in the literature, low BMI was not correlated with COPD,3,19,32 and obesity was found to be correlated with restrictive patterns;2 the nonsignificant trend found in this present study could be explained by a low power of the study to detect BMI difference or by the subjective nature of weight and height reporting in this study.
The present study may be subject to several biases: a selection bias is possible due to the nature of the selected sample. Although a weighting procedure was used to make the sample as representative of the general population in Lebanon as possible, there was still a possibility of a selection bias because there was no means to evaluate the reasons of refusal, which could have lead to an overestimation or underestimation of the COPD prevalence. The authors of this study guess that refusals were linked to nonmotivation, illiteracy, or maybe due to chronic diseases that individuals did not want to disclose (cancer or other diseases). However, the low refusal rate obtained makes this issue of minor importance. There is also a possibility of an information bias in this study: as in all studies involving questionnaires, relying on individuals reporting for several variables may include a recall bias (for previous smoking data for example) or subjectivity bias (for weight, height, symptoms reporting, and other reported information in the questionnaire). Moreover, the portable spirometer used may not be as sensitive and specific as the ones that are used in hospitals, which may introduce a classification bias. However, the methods used in the study are the ones that are currently being used all over the world for this kind of study; in the future, improved methods may help to fine tune the results of this particular study, but the authors believe that changes will not be of major importance.
Conclusion
In this first epidemiological study in Lebanon about COPD, a high prevalence of the disease was determined, 80% of which was undiagnosed by a physician. Older individuals have an increased risk of COPD, in addition to “ever” cigarette smokers and water-pipe smokers. Older age, duration and frequency of cigarette smoking, and duration of previous water-pipe smoking correlated with severity of obstruction. Awareness must be raised about COPD in Lebanon, and diagnosis must be improved. Treatment and prevention programs, added to smoking-cessation programs tackling the unmasked risk of water-pipe smoking,33 must be implemented to reduce prevalence, morbidity, and mortality from COPD.
Acknowledgments
Mirna Waked made substantial contributions to conception and design, acquisition of the data, and interpretation of the data. She also drafted the submitted article and provided final approval of the version to be published.
Georges Khayat made a substantial contribution to analysis and interpretation of the data. He also revised the submitted article critically for important intellectual content and provided final approval of the version to be published.
Pascale Salameh made substantial contributions to conception and design, acquisition analysis, and interpretation of the data. She drafted a part of the submitted article, revised the article critically for intellectual content, and provided final approval of the version to be published.
This study was supported by an unrestricted educational grant from Boehringher Ingelheim Pharmaceuticals, which had no role to play in the whole concept, neither in the design nor in the course of the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease:
GOLD executive summary. Am J Respir Crit Care Med. 2007;176: 532–555.
2. Ohar JA, Sadeghnejad A, Meyers DA, Donohue JF, Bleecker ER. Do symptoms predict COPD in smokers? Chest. 2010;137:1345–1353.
3. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012.
4. Vollmer WM, Gíslason T, Burney P, et al. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J. 2009;34:588–597.
5. Agusti A, Calverley PMA, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122.
6. Vas Fragoso CA, Gill TM. Defining chronic obstructive pulmonary disease in an aging population. J Am Geriatr Soc. 2010;58(11):2224–2226.
7. Swanney MP. Lower FEV1 limit aids airway obstruction classification. Thorax. 2008;63:1046–1051.
8. Celli BR. The light at the end of the tunnel: is COPD prevalence changing? Eur Respir J. 2010;36:718–719.
9. Cerveri I, De Marco R. What makes large epidemiological studies comparable. Eur Respir J. 2010;36:720–721.
10. MOSA Ministry of Social Affairs and Central Administration of Statistics. The National Study for Households Living Conditions in 2007. Beirut, 2008. Available from: http://www.cas.gov.lb.
11. Central Administration of Statistics. Index of circumscriptions, villages and cities in Lebanon. Jun 2005, Beirut, Lebanon. Available from: http://www.cas.gov.lb
12. Pellegrino R, Viegi G, Brusasco V et al. Series ‘ATS/ERS task force: standardization of lung function testing’. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968.
13. Ferris BG. Epidemiology standardization project. Am Rev Resp Dis. 1978;118:1–88.
14. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586.
15. Soriano JB, Ancochea J, Miravitlles M, et al. Recent trends in COPD prevalence in Spain: a repeated cross-sectional survey 1997–2007. Eur Respir J. 2010;36:758–765.
16. Caballero A, Torres-Duque CA, Jaramillo C, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude. Chest. 2008;133:343–349.
17. Rumeau-Roquette C, Breart G, Padieu R. Methods in epidemiology: sampling, investigations, and analysis. Paris, France: Flammarion; 1985:71–82. [French].
18. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319.
19. Bridevaux P-O, Probst-Hensch NM, Schindler C, et al. Prevalence of airflow obstruction in smokers and never-smokers in Switzerland. Eur Respir J. 2010;36:1259–1269.
20. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (The BOLD Study): a population-based
prevalence study. Lancet. 2007;370:741–750.
21. Vasankari TM, Impivaara O, Heliövaara M, et al. No increase in the prevalence of COPD in two decades. Eur Respir J. 2010;36:766–773.
22. Mannino DM, Watt G, Hole D, et al. The natural history of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:627–643.
23. Hanania N, Celli B, Donohue J, et al. Bronchodilator Reversibility in COPD. Chest. 2011;140(4):1055–1063.
24. Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180.
25. Varela MVL, Montes de Oca M, Halbert RJ, et al. Sex-related differences in COPD in five Latin American cities: the PLATINO study. Eur Respir J. 2010;36:1034–1041.
26. Partridge MR, Miravitlles M, Ståhl E, Karlsson N, Svensson K, Welte T. Development and validation of the capacity of daily living during the
morning questionnaire and the global chest symptoms questionnaire in COPD. Eur Respir J. 2010;36:96–104.
27. Weatherall J-M, Marsh S, Shirtcliffe P, Williams M, Travers J, Beasley R. Quality of life measured by the St George’s respiratory questionnaire and spirometry. Eur Respir J. 2009;33:1025–1030.
28. Burgel PR, Paillasseur JL, Caillaud D, et al. Clinical COPD phenotypes: a novel approach using principal components and cluster analysis. Eur
Respir J. 2010;36:531–539.
29. Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959.
30. Raad D, Gadda S, Schunemann HJ. Effects of waterpipe smoking on lung function: a systematic review and meta-analysis. Chest. 2011;4:764–774.
31. Waked M, Salameh P, Aoun Z. Water-pipe (narguile) smokers in Lebanon: a pilot study. East Mediterr Health J. 2009;15(2):432–442.
32. Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–763.
33. Chan A, Murin S. Up in smoke: the fallacy of the harmless hookah. Chest. 2011;139:737–738.